Introduction to Megakaryopoiesis
In simple words, the process of Megakaryocyte formation is called Megakaryopoiesis. It is a complex and stepwise process in which a megakaryocyte produce, differentiates and further grows, and matures inside the bone marrow.
On the other hand, thrombopoiesis is the process of platelet formation which is a later process. These processes are carried out by various growth factors and hormones in successive lineage commitment steps to generate megakaryocytes (MKs).
The very slight quantity of megakaryocytes (0.1%) of total nucleated cells inside the bone marrow produces about a billion platelets per day and this quantity may reach an average of 15 folds during disease or by exogenous thrombopoiesis-stimulating drugs i.e. Romiplostim.
Just like all other blood cells, megakaryocytes are also produced in the bone marrow from Pluripotent Hematopoietic Stem Cells (PHSC). As shown in the following hematopoietic lineage figure.
Overview of Magakariopoisis
The (PHSC) is differentiated into Erythroid and Myeloid progenitors (CFU-GEMM). It is also called common myeloid progenitor cells or myeloid stem cells. “GEMM” stands for granulocyte, erythrocyte, monocyte, and megakaryocyte. The myeloid-erythroid progenitor (MEP) makes a precursor cell for the formation of the Megakaryocytes called the committed progenitor, which has similarity with the lymphoid cells in the apparent structure.
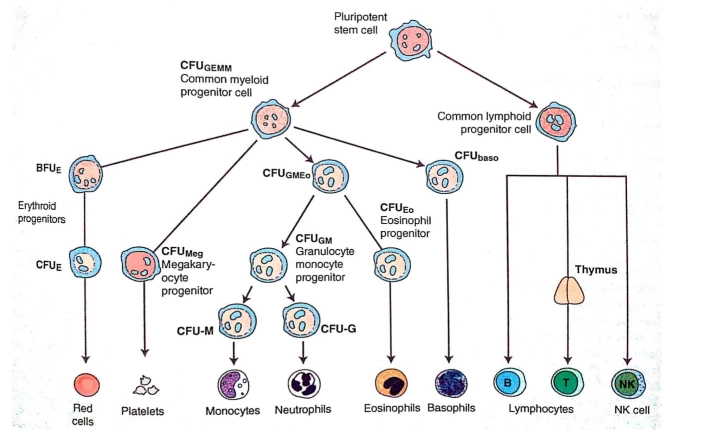
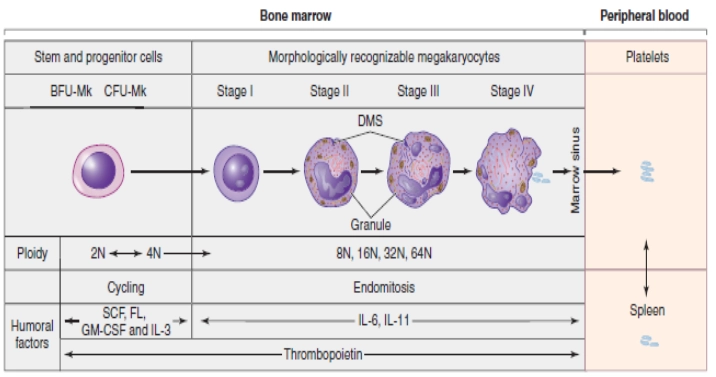
As shown in the figure above, the Megakaryocyte committed progenitor cell gives rise to BFU-MK which are burst forming units, Meg-CFC or CFU-MK. The (CFU-MK) is a cell that develops into a simple colony containing from 3 to 50 mature megakaryocytes while other more complex colonies that include satellite assembly of megakaryocytes and have hundreds of cells are derived from the burst-forming unit–megakaryocyte (BFU-MK). Due to the difference in their proliferative potential, BFU-MK and CFU-MK are thought to represent the primitive and mature progenitors restricted to the lineage, respectively. The time taken by the whole process from the MEP to the formation of the platelet is about a week.
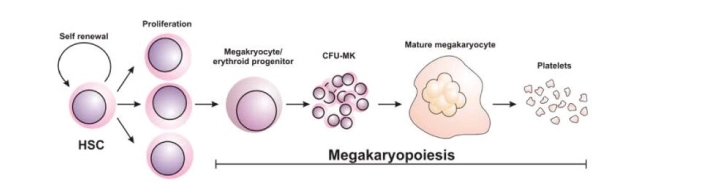
Megakaryocytes are found primarily in the vascular aperture where they physically attach to endothelial cells lining the sinusoidal vessels. Marrow stromal cells produce both positive and negative regulators of megakaryocyte growth factors. Stromal Cell-Derived Factor-1 (SDF-1) also contributes to megakaryopoiesis by enhancing thrombopoietin (TPO) induced megakaryocyte growth and endothelial adhesion within the marrow microenvironment. TPO is the most important signal to stimulate the growth of about 75% of the CFU-MK and sometimes IL-3 alongside the TPO for the remaining 25% of the proliferation and growth.
Developmental Stage of Megakaryopoiesis
As mentioned earlier that the process of megakaryopoiesis is carried out by various chemokines but among them, the cytokine TPO, IL-3, and IL-6 are the major regulators of megakaryopoiesis. There are 4 important and highlighted steps in which MK progenitors proceed to the final maturation.
The earliest cytochemical recognizable cell (by their expression of megakaryocyte specific markers) of the megakaryocyte progenitor lineage is Megakaryoblast. During this stage, MKs undergo repeated incomplete cell cycles in which mitosis is absorbed in late anaphase with the absence of karyokinesis and cytokinesis, and is known as endomitosis.
In other words, it is a unique form of mitosis in which the cell’s DNA content doubles, but cell division and nuclear division do not take place. Endomitosis results in an increase in the DNA contents and finally polyploidy occurs in which the DNA is an exact multiple of the normal 2n. It may reach from 2n to 128n but 16n is a common polyploidy stage in adult human beings. The cytoplasmic maturation takes place normally at the stage of 8n. (Tianyu Guo, 2015)
The development of the megakaryocyte is divided into some stages on the basis of the quantity and characteristics of the cytoplasm, size, lobulation, and chromatin pattern of the nucleus. These stages are elicited as under.
Stage 1: (Megakaryoblast)
The cell in this stage is 6-24 microns in diameter. It has scant basophilic cytoplasm with no visible granules, round nuclei, and visible nucleoli. In this stage, the cell is called Megakaryoblast.
Stage 2: (Promegakaryocyte)
The cell in this stage is 14-30 microns in diameter. Larger cytoplasm than Megakaryoblast and is primarily basophilic. Few azurophilic cytoplasmic granules can be seen. The nucleus is bilobed or intended and membrane demarcation starts. Cells in this stage are also called basophilic megakaryocytes.
Stage 3: (Granular Megakaryocyte)
The diameter of the cell in this stage is about 25-50 microns. Abundant acidophilic cytoplasm with numerous granules, multilobed nucleus, and no visible nucleoli.
Stage 4: (Mature Megakaryocyte)
In this final developmental stage of megakaryopoiesis, the diameter of the cell is 40-100 microns, with abundant acidophilic and very granular cytoplasm, multilobed nucleus, and no visible nucleoli. A Demarcation zone is present in this stage.
Platelets production or Thrombopoiesis
After the successful megakaryopoiesis, there is a later mechanism of platelet production which was a matter of scientific conflict and research hotspot for several decades. However, the study of recent years, scientists now broadly accept that platelets are released from MKs through the formation of proplatelets from mature MKs as an intermediate before terminal platelet production.
Proplatelets are extensions of MK’s cytoplasmic processes, terminating in small‐sized outgrowths. A demarcation membrane system (DMS), makes the membrane of the proplatelets. This membrane is a large invagination that has a continuation with the surface of the megakaryocytes. The range of this membrane is directly related to the number of proplatelets released for an MK. (Avanzi, M. and Mitchell, W., 2014)3
Polyploidization and maturation of megakaryocytes were observed to have vital roles in the number of platelets released per cell. The microenvironment of the perivascular niche provides external stimuli including fibronectin and fibrinogen.
The only, very important, and final factor in platelet production is the shear stress within the sinusoid vessels in the marrow. Shear stress alone has the ability to initiate and induce elongation of the cytoplasm and shedding of platelets, but this effect is tremendously enhanced in the presence of ECM proteins. Apoptosis may also play a role in the formation of proplatelets, but the exact mechanism is unknown yet. (Avanzi, M. and Mitchell, W., 2014)
Qualitative platelets disorders
Qualitative disorders of platelets involve structure or functional abnormality due to missing or defective proteins on the surface of the platelets membrane or there may be an abnormality in granules of platelets. These abnormalities may be inherited or acquired. (Melissa Gomez, 2020)
E.g.
• Bernard-Soulier syndrome (inherited)
• Glanzmann Thrombasthenia (inherited)
• Myeloproliferative diseases (acquired)
• Myelodysplasia (Acquired)
• Acute leukemia (Acquired)
• Monoclonal gammopathy (Acquired)
• Cardiopulmonary bypass platelet trauma (acquired)
Besides all these above-mentioned disorders, sometimes there are complications that are drug-induced, they are separately considered but can be linked with the acquired qualitative platelets disorders. (Dominique, W., 2016)
Bernard – Soulier syndrome
In this disease, there is a dysfunction of blood platelets. This is categorized by thrombocytopenia but also magathrombocytes and high bleeding disposition. In other words, it can be said that the number of platelets is not normal but low and the still the percentage of platelets is high. (Melissa Gomez, 2020)
Etiology
Modern researches have that, there is a deficiency of a single glycoprotein on the surface of the thrombocyte which is needed to bind to Von Willebrand factor (VWF) having a vital role in the normal process of clotting. The disease is genetic (autosomal recessive), and two copies of the gene are required to declare a disorder.
Glanzmann Thrombasthenia
It is also a bleeding disorder that is characterized by long-lasting or sometimes spontaneous bleeding starting from infancy. People with this disease usually have bleeding of the nose (epistaxis), and gum bleeding.
Etiology
The major cause of this disease is mutations of ITGA2B or ITGB3 genes. These genes code for two subunits of a receptor protein known as integrin alpha-IIb/beta3 (αIIbβ3). (Melissa Gomez, 2020)
Other common etiologies or causes of qualitative platelets disorders:
• Bone marrow failure
• Infiltration by leukemia
• Malignancy
• Fibrosis
• von Willebrand disease
• Drugs ( e.g. aspirin, NSAIDs, alcohol)
• Uremia
• Paraproteins
• Fibrin degradation products
• Myelodysplasia or a myeloproliferative syndrome (Thiagarajan, 2020)
Quantitative platelets disorders
Quantitative platelet disorders are refed to as the number of circulating platelets is not enough or very much high, it may due to insufficient production of platelets or the abnormal destruction of platelets. (Melissa Gomez, 2020)
Some common quantitative platelet disorders are mentioned as under. There are also two major categorizations of quantitative platelet disorders on the basis of their etiology. They may be congenital or acquired.
A. congenital thrombocytopenia includes the following most important diseases.
• Autoimmune lymphoproliferative syndrome
• Congenital amegakaryocytic thrombocytopenia
• Fanconi anemia
• Wiskott-Aldrich syndrome
(Melissa Gomez, 2020)
B. The acquired quantitative platelets disorder includes the following important disorders.
• Drugs & alcohol
• Nutritional deficiency: such as vitamin B12, Folate
• Infiltrative disease
• Viral: HIV, Hepatitis
(Melissa Gomez, 2020)4
In the increased destruction category, immune-mediated thrombocytopenia is the most common which includes the following important disorders.
• Immune thrombocytopenia
• Neonatal alloimmune thrombocytopenia
• Acquired aplastic anemia
• Thrombocytopenia induced by toxins
• Immune thrombocytopenic purpura (ITP)
• Cyclic thrombocytopenia
• Thrombocytopenia after blood transfusion
(Melissa Gomez, 2020)
Note: Thrombocytosis has been excluded from all the above-mentioned disorders. This disease is primarily indicated by the elevated amount of platelets in the blood.
Post Operative thrombocytopenia
Postoperative thrombocytopenia is defined as any notable decrease in the number of platelets after surgery (20% or 30%) compared with a preoperative normal count which is between 150000-450000. A platelet count decrease from the normal range is expected within 4 days of surgery and is more prominent on 1st or second day of cardiac surgery. As it is concluded that, almost all patients undergoing major surgery will experience this problem.
(Skeith, Baumann, 2020)
The contrasting findings of postoperative thrombocytopenia show pseudo thrombocytopenia (which may include platelet clumping or platelet satellitism), hemodilution, elevation, or depression in production, clearance, and destruction. (Skeith, Baumann, 2020)7
Pathological causes of Postoperative thrombocytopenia:
It cannot be said that a patient prior to surgery, has no thrombocytopenia related to already present bone marrow disorder, but on the other hand, sudden postoperative thrombocytopenia (POTP) may be more fetal and life-threatening but may not be evident.
Unanticipated (POTP) is caused by heavy platelet use or platelet demolition. Platelet heavy use refers to those disorders which are characterized by pathologically accelerated normal physiological processes, i.e. thrombin g or platelet generation, (VWF) von Willebrand factor interactions in disseminated intravascular coagulation (DIC) and sometimes thrombotic microangiopathies (TMAs).
On the other hand, Platelet demolition refers to those processes which are non-physiological including direct immune-mediated platelet clearance (DIMPC), as observed in drug-induced ITP (DITP), immune thrombocytopenia (ITP), or posttransfusion purpura (PTP), among many others. (HIT) Heparin-induced thrombocytopenia is a distinctive prothrombotic disorder in which antibody-mediated activation of platelets occurs with collaterally marked coagulation activation.
(Skeith, Baumann, 2020)
Postoperative management/treatment of thrombocytopenia:
Studies show that the effects of blood transfusion may lead to smaller increases in mortality and complications in thrombocytopenic patients. Although, we could not precisely find the effect of blood transfusion on the adverse effects of thrombocytopenia because there is no detailed information on postoperative blood transfusion. (Glance et al., 2014)8
Other managements include.
• Steroids
• Immune globulins
• Drugs that boost platelet production i.e. Romiplostim, Promacta, etc.
• Other drugs i.e. Rituximab, Troxima, etc.
Summary of Magakaryopoiesis
Platelets are cells that are required for the normal clotting purpose to prevent bleeding, these cells are produced in the bone marrow by a very long and complex process. First, the stem cell of the bone marrow is committed to producing a mature cell called a Megakaryocyte by a process called megakaryopoiesis.
After this very process, there is the formation of platelets from the fragmentation of the cytoplasm of the megakaryocytes. There are four major important steps of megakaryopoiesis. In the first step, the cell is called Megakaryoblast then promegakaryoblast then granular megakaryocyte, and finally mature megakaryocyte. This process is carried out under a series of activation factors and hormones i.e. TPO, IL-3, and IL-6, etc.
Sometimes alteration may occur in the platelets production or its use which then make a great deal of complication i.e. thrombocytopenia and thrombocytosis. Some of the platelet defects are qualitative while others are quantitative, both can make problems in normal human physiology. Sometimes complications may occur after a surgical procedure and thrombocytopenia may occur which is very challenging. These complications are managed in a proper way.
References
- Kaushansky, K., 2008. Historical review: megakaryopoiesis and thrombopoiesis. Blood, 111(3), pp.981-986
- Tianyu Guo, Q., 2015. Megakaryopoiesis And Platelet Production: Insight Into Hematopoietic Stem Cell Proliferation And Differentiation. [online] PubMed. Available at: https://pubmed.ncbi.nlm.nih.gov/27358871/ [Accessed 3 January 2021].
- Avanzi, M. and Mitchell, W., 2014. Ex Vivoproduction of platelets from stem cells. British Journal of Haematology, 165(2), pp.237-247
- Melissa Gomez, M., 2020. 5+ Quantitative & Qualitative Platelet Disorders. [online] Thrombocyte.com. Available at: https://www.thrombocyte.com/ [Accessed 4 January 2021]
- Dominique, W., 2016. Qualitative Platelet Disorders | Washington Manual Of Medical Therapeutics. [online] Unboundmedicine.com. Available at: https://www.unboundmedicine.com/ [Accessed 4 January 2021].
- Thiagarajan, P., 2020. Platelet Disorders: Overview Of Platelet Disorders, Pathophysiology Of Platelet Disorders, Autoimmune Thrombocytopenias. [online] Emedicine.medscape.com. Available at: https://emedicine.medscape.com [Accessed 4 January 2021].
- Skeith, L., Baumann Kreuziger, L., Crowther, M. and Warkentin, T., 2020. A practical approach to evaluating postoperative thrombocytopenia. Blood Advances, 4(4), pp.776-783.
- Glance, L., Blumberg, N., Eaton, M., Lustik, S., Osler, T., Wissler, R., Zollo, R., Karcz, M., Feng, C. and Dick, A., 2014. Preoperative Thrombocytopenia And Postoperative Outcomes After Noncardiac Surgery. https://pubs.asahq.org/.